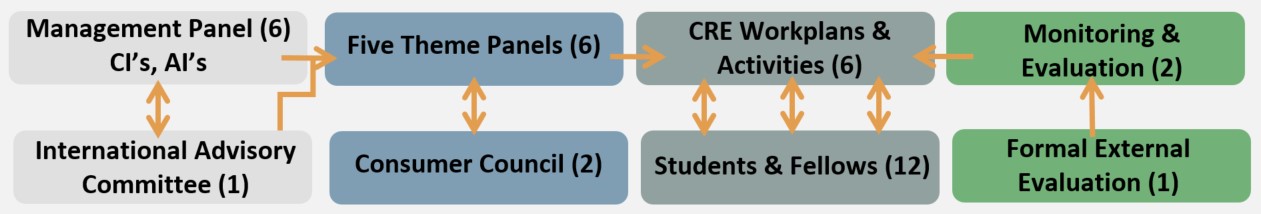
The National Health and Medical Research Council (NHMRC) has granted $2.49 million over five years to establish a Centre for Research Excellence (CRE) to improve health outcomes for children with cerebral palsy.
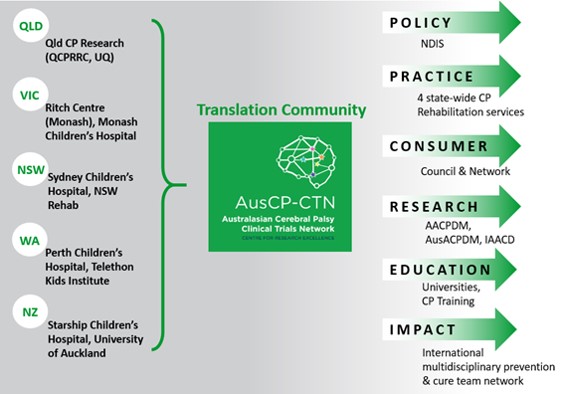
Cerebral Palsy (CP) is the most common physical disability of childhood (≈38,000 cases in Australia, 450 new cases p.a.). It is caused by an early brain injury where 70% of cases occur in the 3rd trimester of pregnancy. Despite the early brain injury, children with CP are not diagnosed until on average 19 months post term so that only half receive rehabilitation before 1-year of age, meaning the period of neuroprotection and early rehabilitation to optimise brain plasticity is misspent. As CP is an early brain injury, there is greater opportunity to mitigate the brain injury. Our focus is on earlier rehabilitation to maximise adaption of the brain with motor learning focused rehabilitation and neuroprotectants. Our team have identified from clinical trials and meta-analyses the urgent need for novel neuroprotectants, earlier brain repair via cell therapies, and functionally focused rehabilitation that commences earlier than current practice6. This new Clinical CRE builds on the fundamental research in our first Australasian Cerebral Palsy Clinical Trials Network (Aus-CP-CTN, NHMRC CRE 2016-21) across Australia & NZ moving to implementation of new interventions in this second CRE. Our network will conduct world standard, multisite, clinical trials to improve early detection and determine the best interventions and treatments for children with cerebral palsy.
Our Aus-CP-CTN national network has delivered the following outcomes:
- Tracked a 30% reduction in the rate and severity of CP (in country-wide Australia CP Register).
- Trained >1,000 clinicians on gold standard tools (General Movements, GMA and the Hammersmith Infant Neurological Assessment, HINE) so that detection of high risk of CP by 6 months (1 year earlier than current practice) has been implemented into clinical practice nationally.
- New clinical tests (Neurological and GMs assessments) and MRI biomarkers of CP in neonatal period have been identified that will enable detection of CP prior to term equivalent age (TEA).
- Trained >60 researchers and clinicians and >50 consumers on Consumer Engagement.
- Harmonised neuroimaging protocols in infants and children with CP for automated analysis and measurement of neuroplasticity in response to interventions.
- New rehabilitation interventions delivered in the first 12 months of life have shown improved functional outcomes in pilot studies and are now being tested in 13 multisite clinical trials (6 NHMRC funded) of early and intensive interventions.
- Testing of 4 neuroprotectants in human trials (melatonin, EPO, Amnion cells, creatine).
- Commenced large multisite national (KITE-CP, n=1,000) and international (EU Horizon Grant “BORNTOGEtTHERe” across 6 countries) implementation studies of early detection and early intervention.
- Commenced large NHMRC multisite intensive rehabilitation studies in school-aged children with CP (HABITILE, n=130) and participation focused interventions (Participate CP, n=100).
- Completed three International Clinical Practice Guidelines (CPG) on (Early Diagnosis, Early Intervention and Functional Therapy) which are being implemented into practice.
- CIA Professor Roslyn Boyd, Queensland Cerebral Palsy & Rehabilitation Research Centre, The University of Queensland
- CIB Professor Iona Novak, The Cerebral Palsy Alliance, The University of Sydney
- CIC A/Professor Michael Fahey, Monash Children's Hospital, Monash University
- CID Professor Paul Colditz, The University of Queensland
- CIE Professor Rod Hunt, Monash Children's Hospital, Monash University
- CIF Dr Sarah McIntyre, The Cerebral Palsy Alliance, The Univesity of Sydney
- CIG Dr Jurgen Fripp, Australian eHealth Research Centre, CSIRO
- CIH A/Professor Leanne Sakzewski, Queensland Children's Hospital, The University of Queensland
- CII Professor Catherine Elliott, Telethon Kids Institute, Curtin University of Technology
- CIJ A/Professor Suzie Miller, Monash University
Our Mission
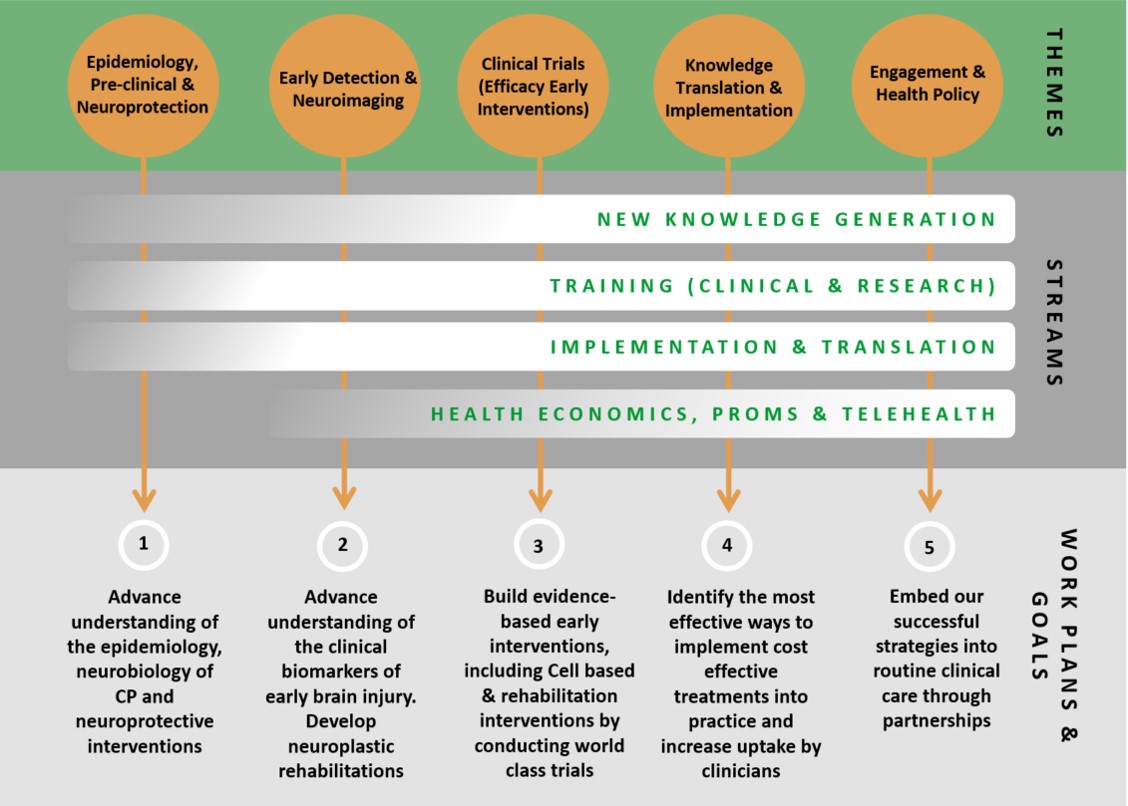
The CRE for the Australasian CP Clinical Trials Network will:
1. Uplift earlier detection of CP.
2. Fast-track children to multisite randomised clinical trials of new neuroprotectants.
3. Develop and test new rehabilitation methods.
4. Ensure new knowledge is transferred effectively to enhance clinical practice by overcoming known barriers to implementation.
5. Develop international clinical practice guidelines guided by a consumer network.